In what might be a foolhardy attempt to correlate two of coffee’s most complicated concepts, I’ve dedicated a significant portion of the past few months to examining the relationship between the water activity of green coffee and the Maillard reaction during roasting.
It’s something my colleague Jen Apodaca and I often discuss when analyzing coffees for Royal’s Crown Jewel lineup: Does water activity affect roasting style? Our colleague over at Red Fox Coffee Merchants, Joel Edwards, has written a bit about it, too. Ironically, of the three major reasons you might measure a coffee’s water activity, deciding on a roasting style is probably the least important.
First and foremost, water activity can tell you about whether the product is safe from being infected by microbes. Higher water activity accurately predicts the growth of molds, yeasts, and bacteria, for example.
Secondly, water activity can help, to some degree, to predict the shelf life of a coffee’s flavors. Coffees with water activity (aW) higher than 0.60 are generally seen as a little more volatile, and in certain transit or storage conditions might begin to taste prematurely aged.
These two metrics are covered in far greater depth elsewhere, including this Green Coffee Analytics article. However, there is a third metric worth mentioning, and it has to do with sugar browning and the Maillard reaction.
A Very Brief Primer on the Maillard Reaction
The Maillard reaction’s importance to food science is impossible to overstate. This sentiment is summed up nicely in a quote by Jean-Marie Lehn, Nobel Prize winning chemist: “The Maillard is, by far, the most widely practiced chemical reaction in the world.”
The Maillard reaction is, in fact, a series of many reactions between sugars and either proteins or amino acids, catalyzed by heat, that involves a lot of molecular disassembly and reassembly. Frankly I don’t comprehend the chemistry behind it all that well, but then again, neither did the French scientist for whom it’s named, Louis-Camille Maillard. After his initial paper in 1912, it took the scientific community more than 50 years to get an accurate model created. Notably, American chemist John E. Hodge was able to “establish a mechanism” that adequately explained it in 1953.
Ultimately, what the Maillard reaction contributes to roasted coffee is complexity of flavor — through the types of sugars, the amount of perceived acidity, and the structure of the viscosity. The Maillard reaction is responsible for nothing short of the development of coffee’s essential sensory character.
Small changes in heat application during Maillard — which usually begins at around 300F and continues through first crack and post-crack sugar browning — can create a dramatic shift in flavor profile. Rob Hoos has published a book about this. His unified theory has to do with longer times spent during Maillard correlating with an increase in perceived viscosity.
The other side to this discussion is worth highlighting, as well. I think there is ample evidence that, all other things being equal, shorter Maillard duration during coffee roasting is related to an increase in the perception of sweetness and acidity.
I suspect this perception is related to available soluble compounds in the roasted coffee. Longer roasting times, as I wrote about a few months ago, seem to decrease solubility in coffees. Among those soluble compounds are things like organic acids (citric, tartaric, malic, e.g.) that will roast away given the opportunity. We also know that as sugars are exposed to heat they caramelize, forming longer molecular chains that are perceived as less sweet than in their raw, short-chain state. Reducing the exposure of organic acids and sugars to long roasting times, particularly during the Maillard reaction can increase their perceptibility in the cup.
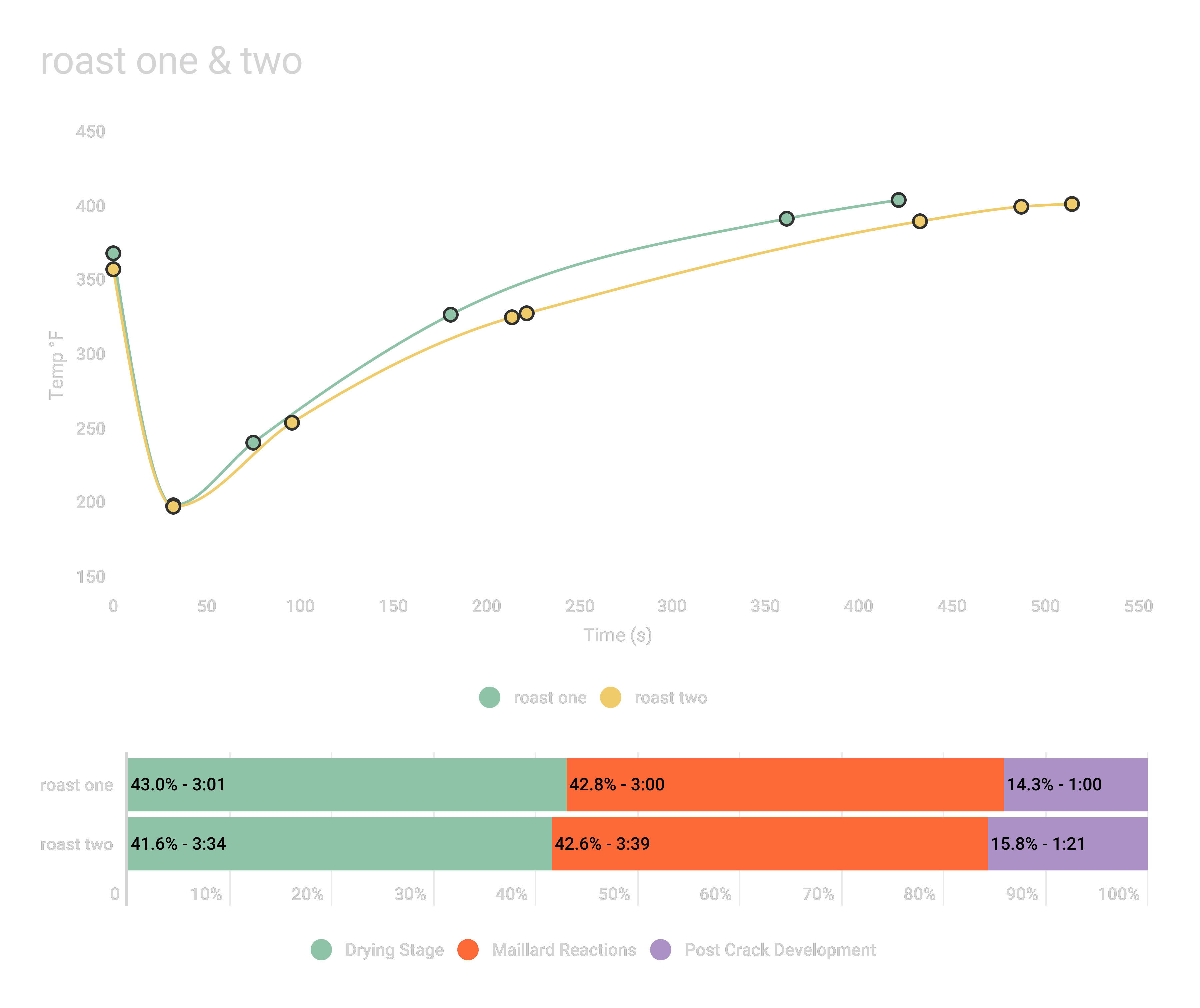
By way of example, here’s a classic case where Jen’s first roast, the green curve below, passes through the Maillard reaction stage more quickly, evidenced by the steeper rate of rise. Flavors included citrus and other bright fruit notes; the coffee was sugary with a thin body. The second roast, by comparison, was mellower: we noted a muted, subtle acidity and a buttery viscosity.
A second example of this principle is nicely illustrated below in a wet-hulled Sumatra, where Jen’s first roast (again in green) had the fastest Maillard reaction rate. Despite 9 seconds longer post-crack development (PCD), 1F higher drop temperature, and a 1 point darker ColorTrack score, it presented notes of lemon, apple, lime, and cherry. The second roast with 28 seconds longer Maillard reaction time (yellow), despite lighter color and shorter PCD, noted complex sugars with an absence of bright fruity notes and a mouth-coating, milky and smooth viscosity.
Relationship Status: Water Activity and the Maillard Reaction
So, what does this all have to do with water activity? Better scientists than I have shown that water activity has the ability to influence both the rate and the color of the Maillard reaction. As water activity approaches 0.70 – in all substances, not just coffee – the rate of the Maillard reaction and sugar browning peaks. Higher than 0.70, Maillard slows because the reactants are diluted by too much free water.
Most coffee above 0.70 is considered less than fully safe from microbial activity and generally unstable for shipping and storage. So, trying to maximize your rate of Maillard reaction during roasting by increasing water activity is discouraged.
However, a coffee’s water activity can inform us a bit about how it is likely to behave during roasting.
First Crack Consistency Case Study
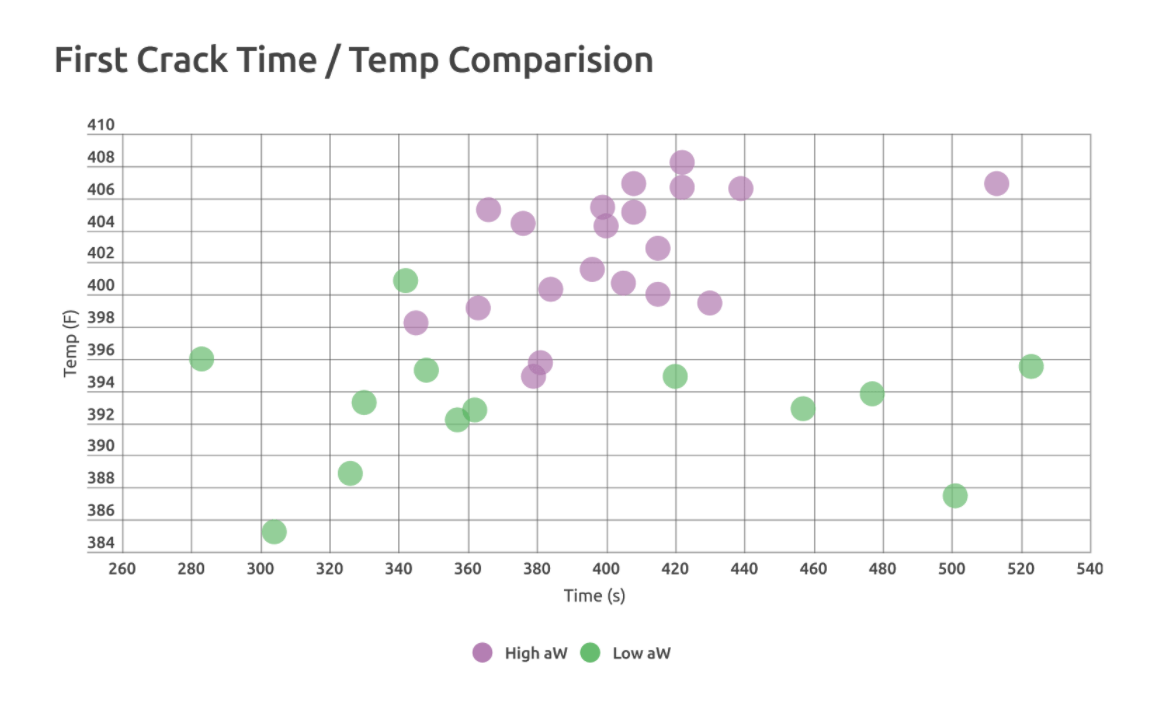
Jen began tracking a really interesting trend in her weekly roasts. She began to correlate the predictability of first crack time and temperature to water activity levels.
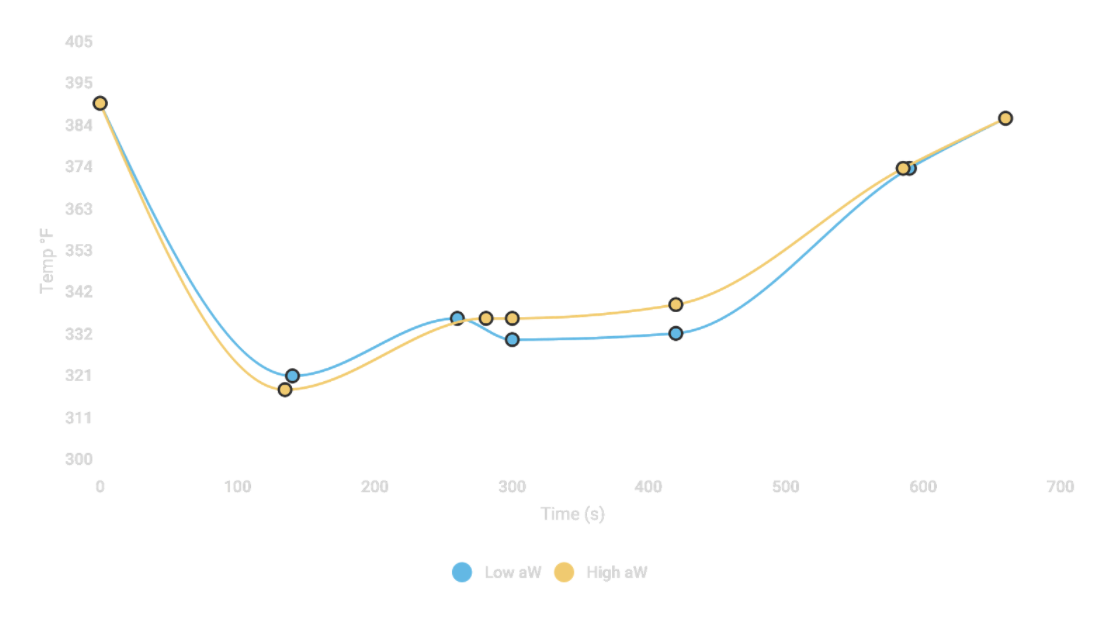
Below, we’ll look at data she gathered from two Guatemalan lots from Huehuetenango, which were harvested and arrived during similar timeframes and had similar moisture content, but different water activity readings. After 34 roasts, in different sizes and on multiple machines in as many as 5 different cities, these comparable coffees showed some remarkable differences. The coffee with higher water activity (~0.58, shown in purple dots on the chart) experienced more consistent first crack temperature and time than did the lower water activity counterpart (~0.54, in green).
Reaction Rate and Roast Color Case Study
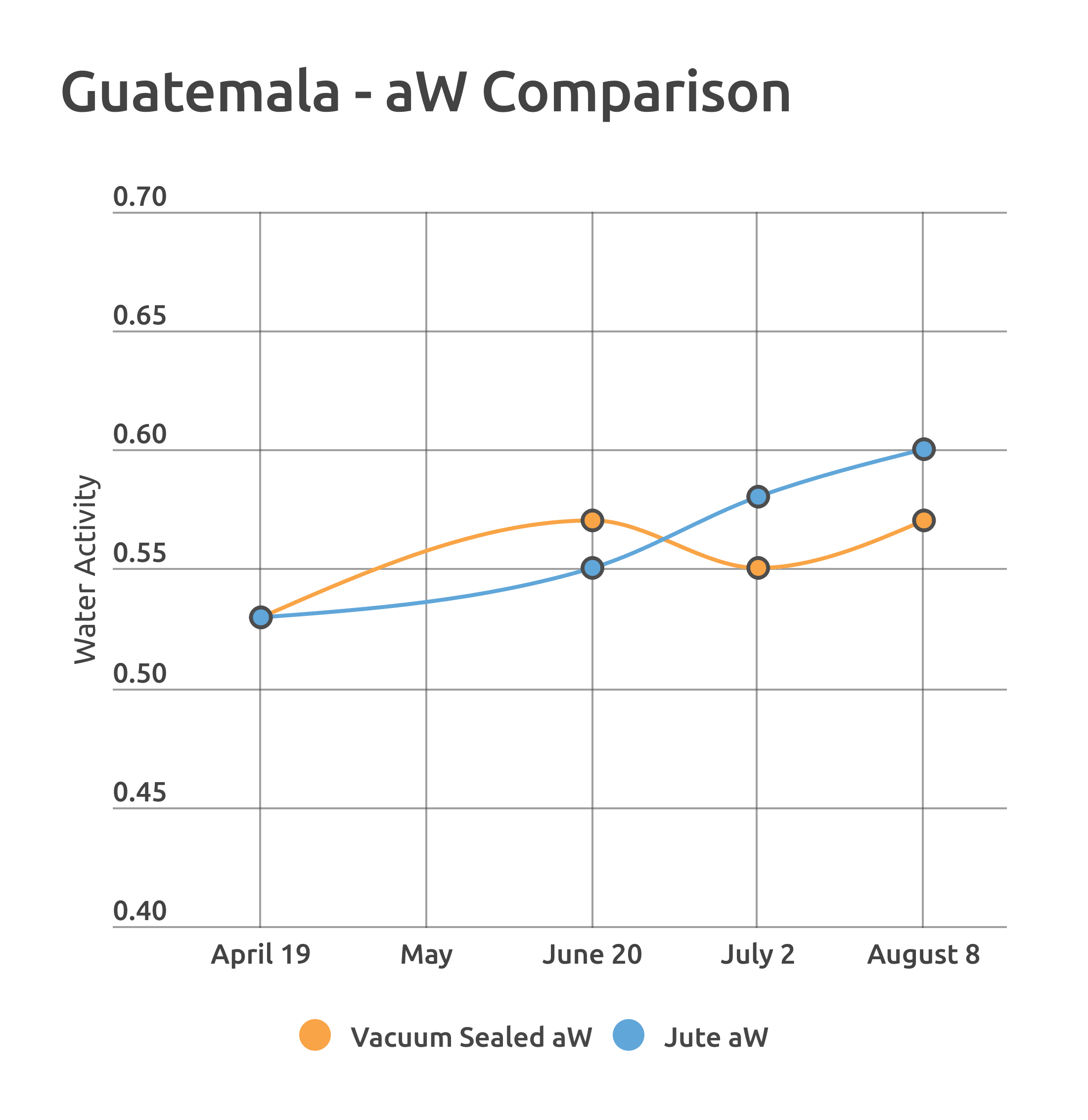
Jen’s work set the stage for isolating a subset to look at closely. I did this in two rounds using a Guatemalan coffee in August, and then a Colombian coffee in October. In both cases, I altered the storage media to affect water activity. In the Guatemalan example, we looked at the long-term differences between jute and vacuum seal repackaging over the course of about 4 months.
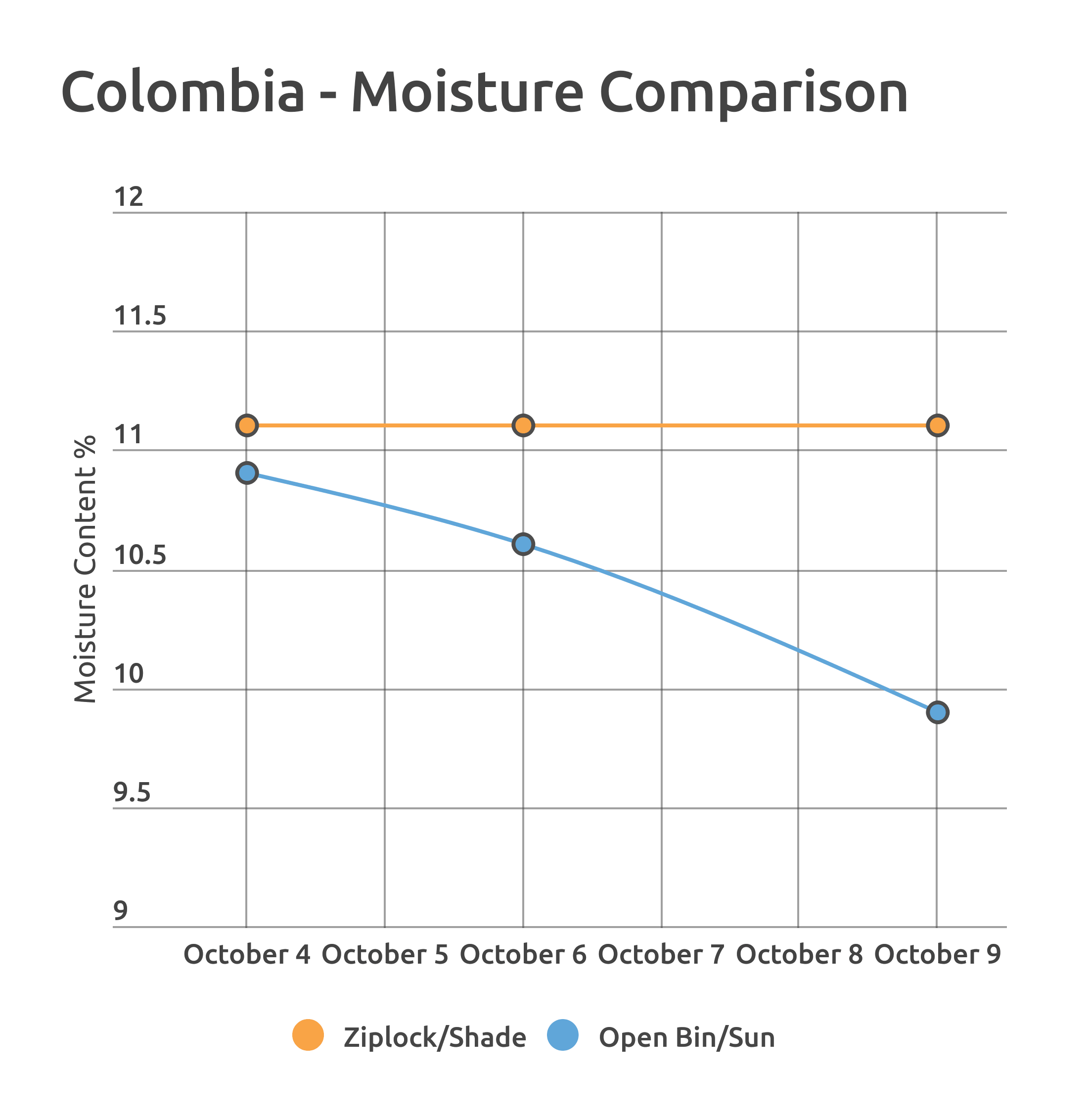
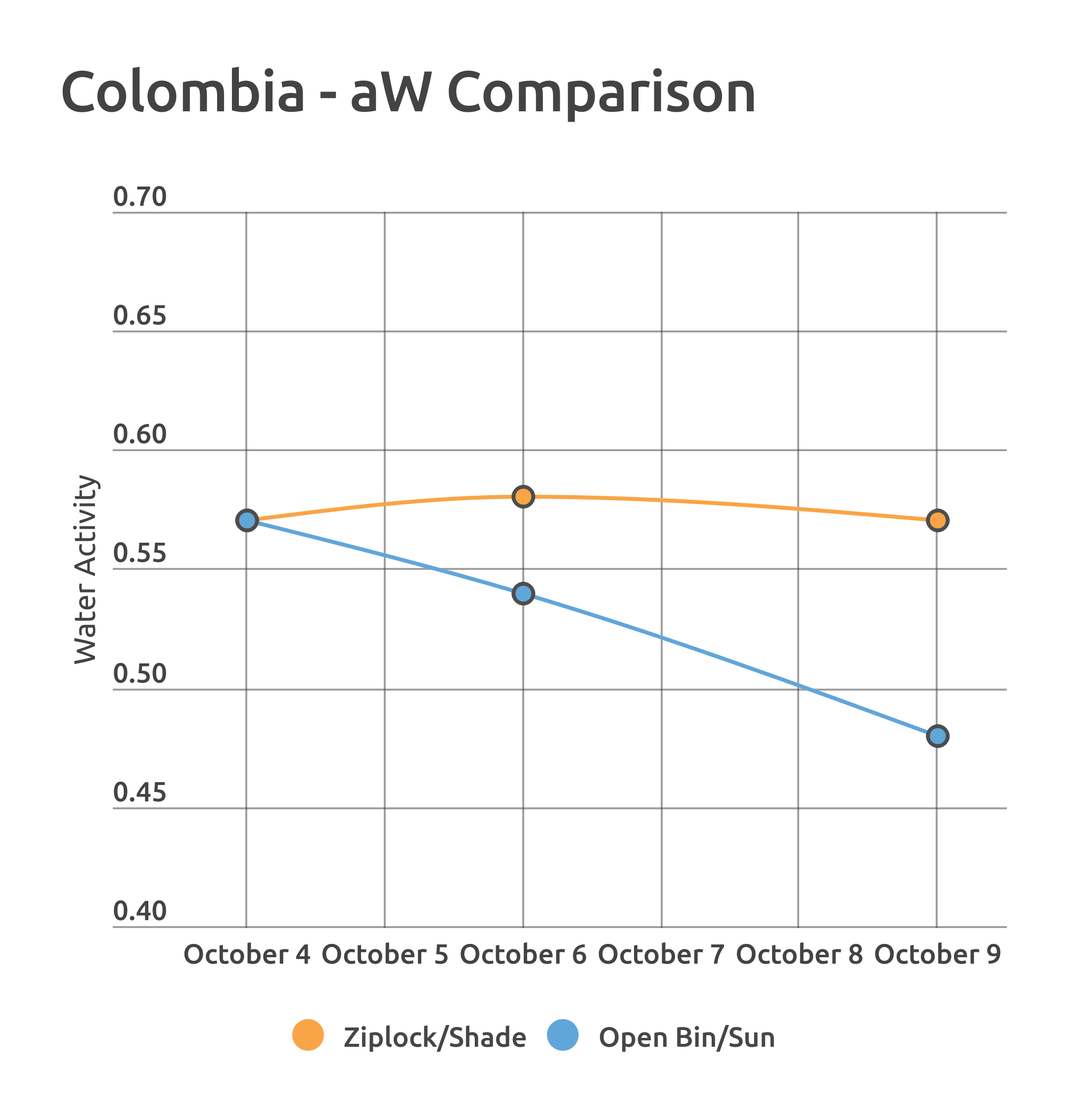
The Colombia was exposed to a more extreme version of repackaging, examining the differences between a shade-stored ziplock bag and an uncovered plastic bin with partial sun exposure. These conditions were over a very short period of time, just five days in total.
The outcome was different for the two coffees, likely the result of the overall length of the experiment. However, it’s worth noting that regardless of the packaging method outcome, there were consistently observed trends related to differences in water activity readings in roasting.
We’ll examine the Guatemala first. In control roasts on a CoffeePro sample roaster, no gas or airflow adjustments were made. The Maillard reaction was marked at ~330F (when visible yellowing is apparent) and first crack was marked at ~385F. Drop temp was contingent on time after first crack, measured at exactly 70 seconds after ~385F.
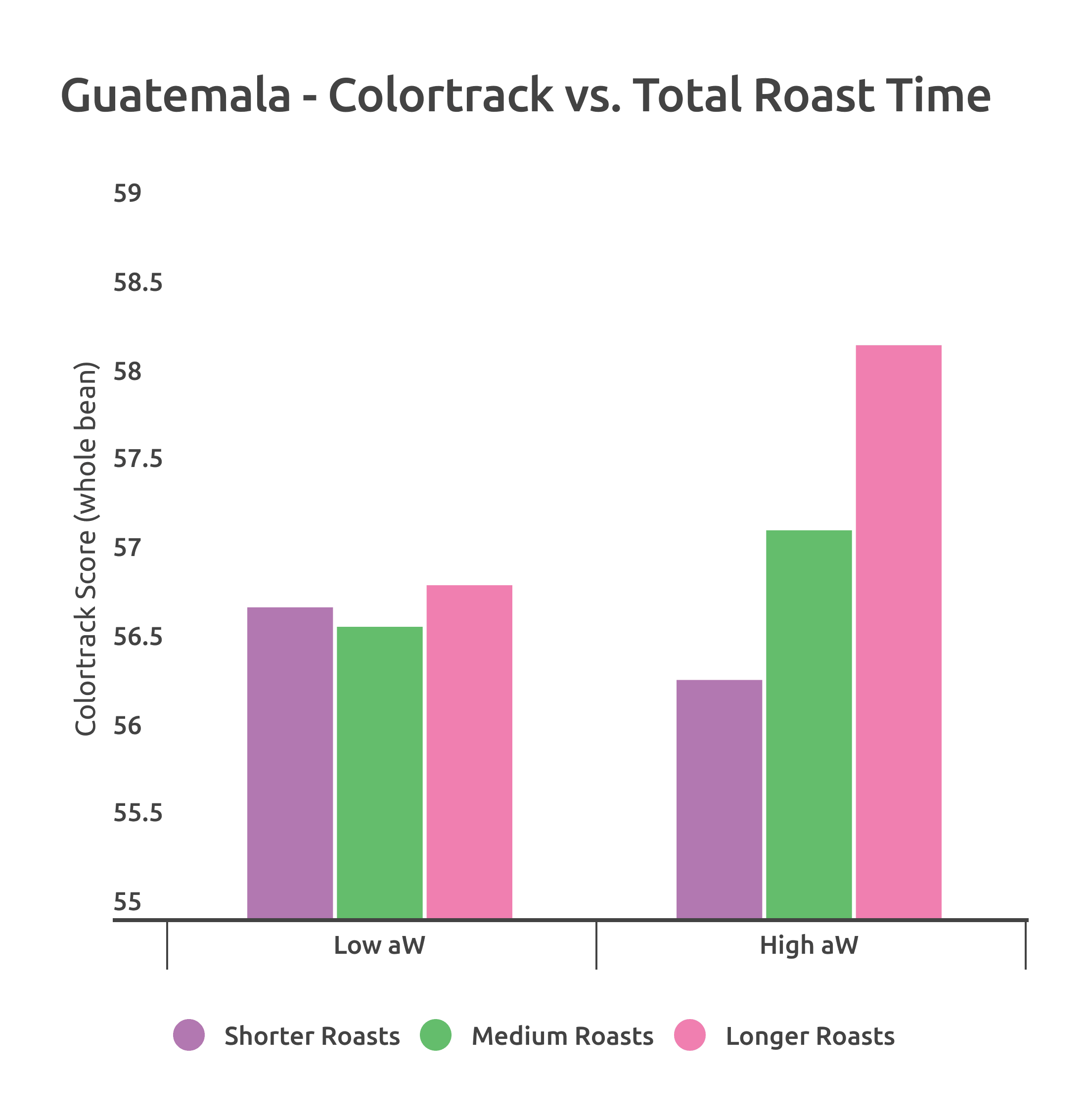
The main difference in these roasts was the relationship of total roast time to whole bean color score, using ColorTrack. Roasts of the High aW sample (yellow) showed more dramatic color change in response to longer roast times compared to the Low aW roasts.
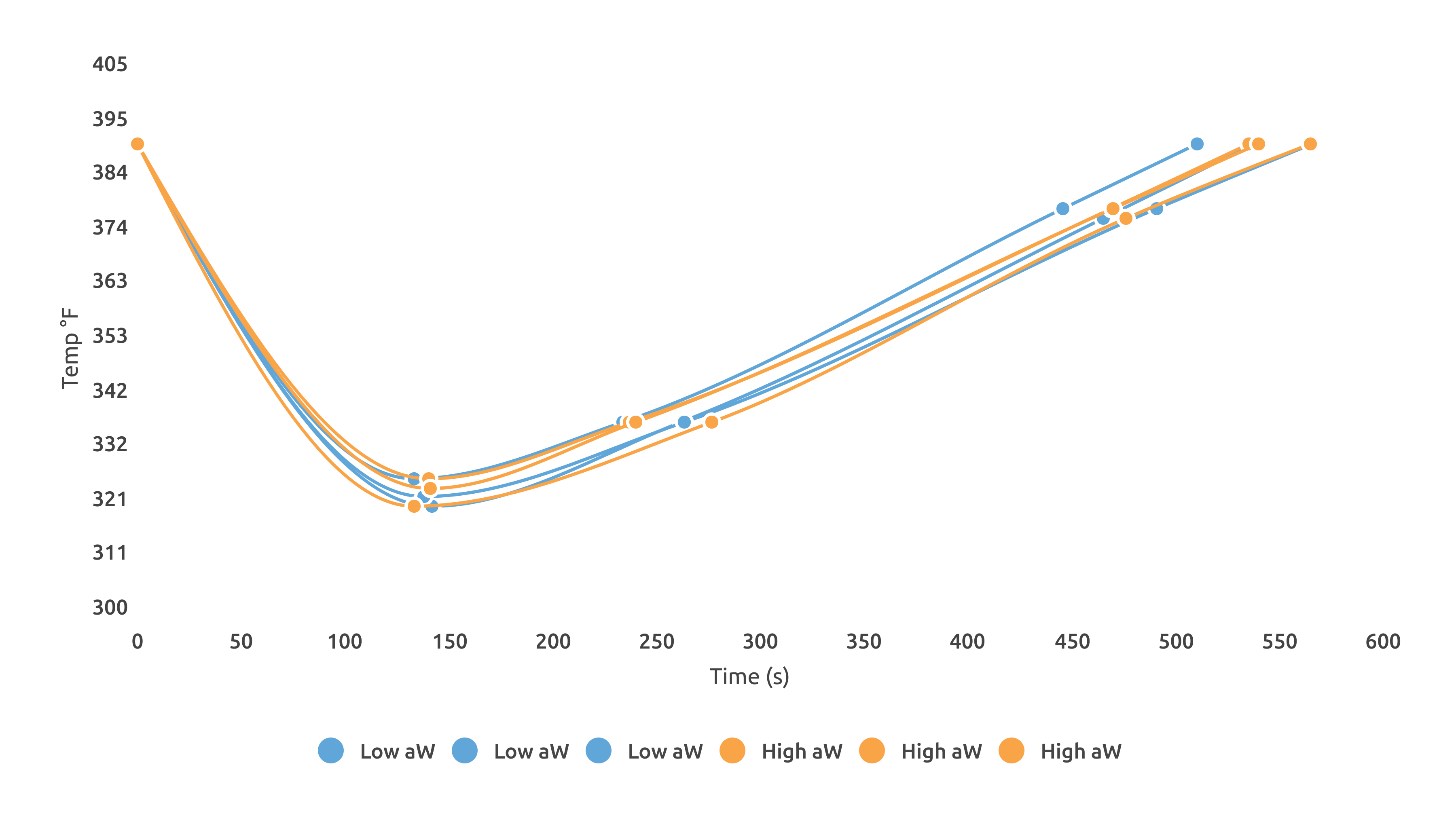
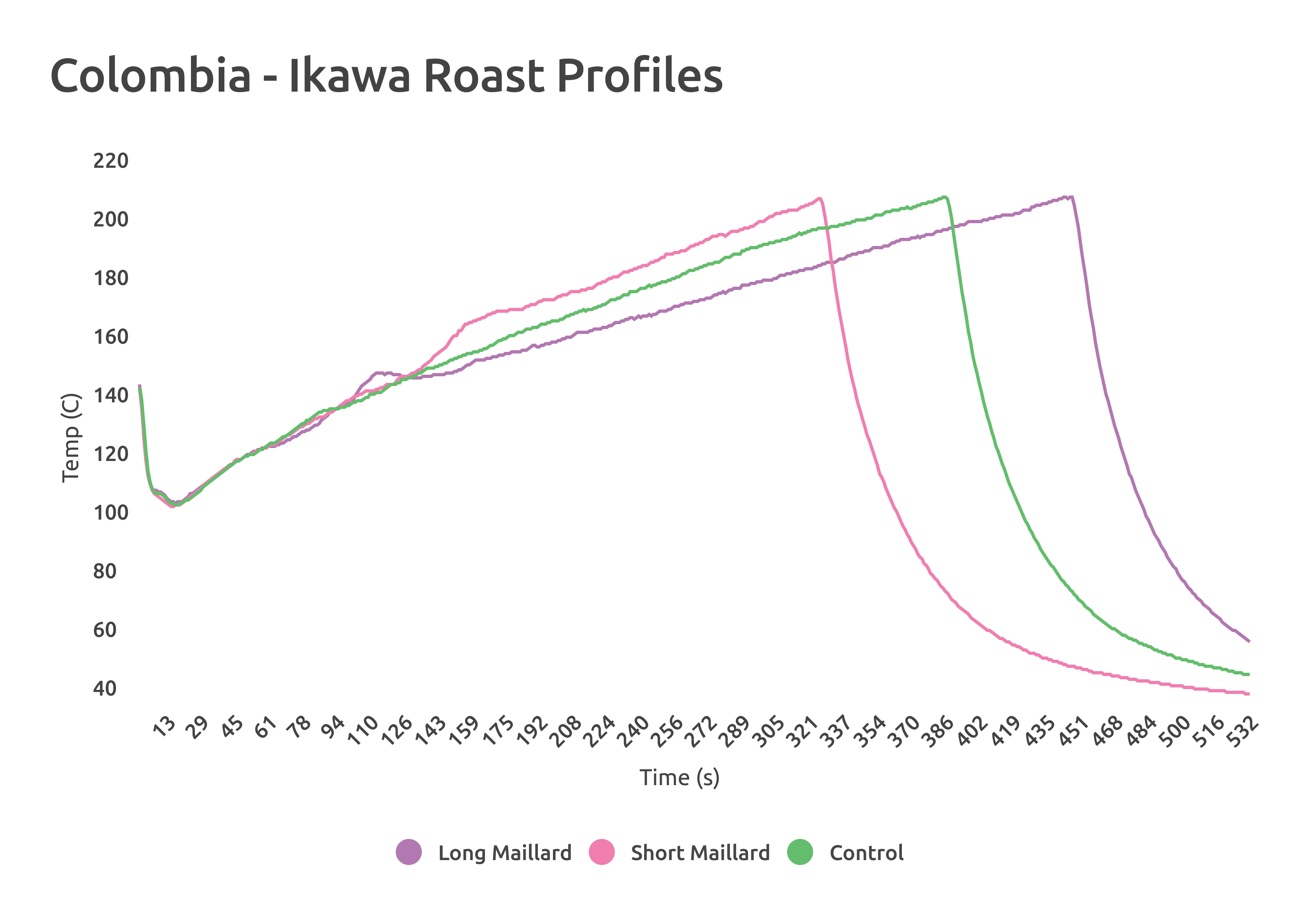
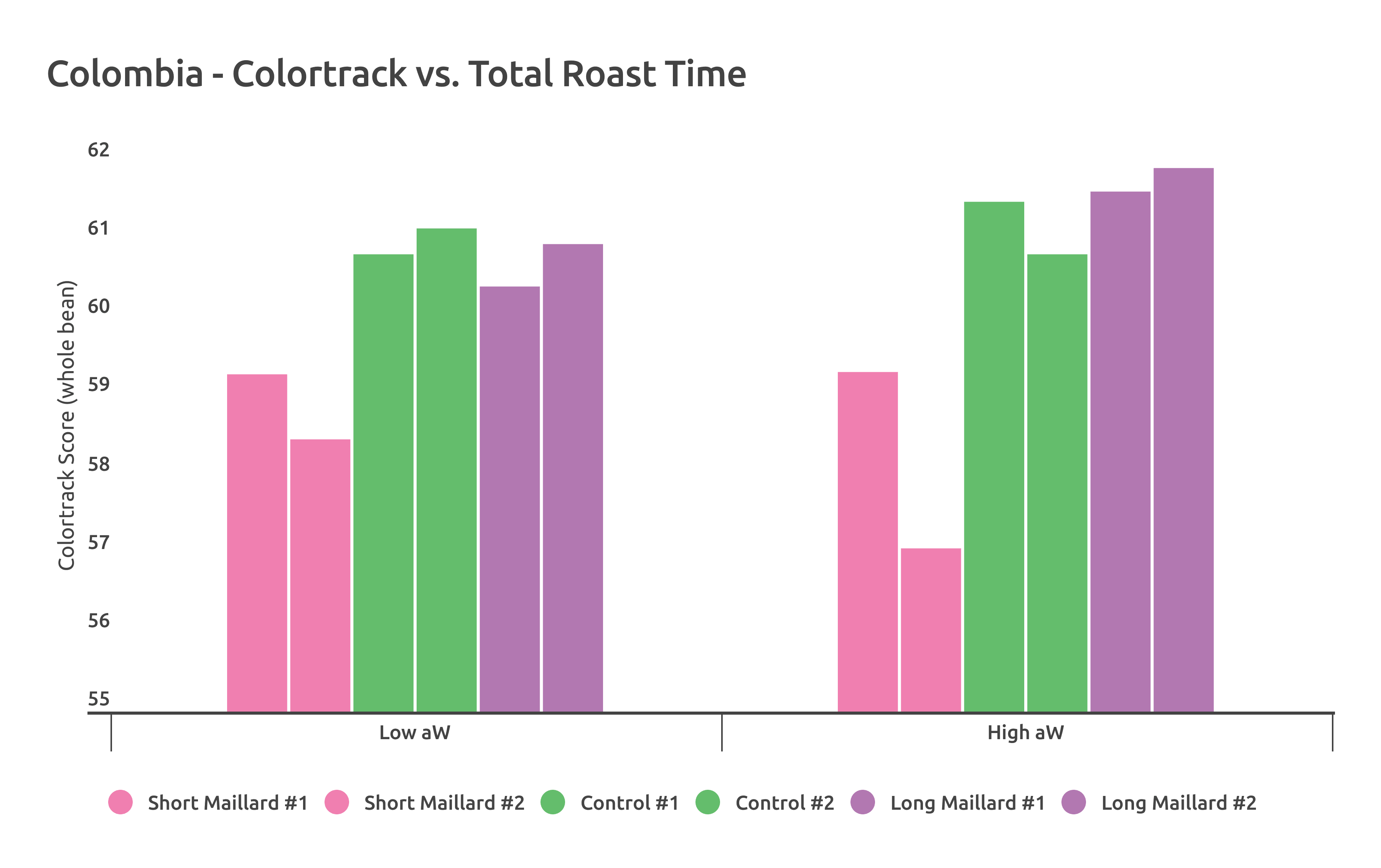
When roasting the Colombia, I used an Ikawa sample roaster to remove the possibility of operator error affecting roast time or temperature. Using 3 different programmed roasts, I changed only the length of time spent moving from 150 C (302 F) to 200 C (392 F) in 1 minute intervals. Because the Ikawa is so precise in its temperature and time programming, no major observable changes in these two metrics were present between the High and Low aW Colombia samples.
Comparing ColorTrack scores for these Colombia roasts on the Ikawa yielded interesting results; more dramatic color change occurred in the High aW sample as a function of length of roast. It also repeated the trend of darker color at longer roasting times for the high water activity sample. The chart below shows whole bean ColorTrack scores of each roast. The samples were roasted at each of the 3 profiles twice.
I’d taken a similar roast style approach on the CoffeePro sample roaster using the Guatemalan coffee in August, using subtle but measurable airflow changes to briefly affect the drum temperature. Increasing the heat had a subtle but measurable impact, where the slow-starting high aW sample increased in temperature more rapidly than its low water activity counterpart. When I decreased the heat at the same time during the roast, the Low aW coffee stalled and dropped about 5 degrees Fahrenheit, but the high aW sample did not.
When I decreased the heat at the same time during the roast, the Low aW coffee stalled and dropped about 5 degrees Fahrenheit, but the high aW sample did not.
One additional observation I made: Regardless of heat application across all the roasts I performed, the Higher aW sample generally had a lower turn-around (minimum) roast temperature.
Crowd-Sourced Sensory Data
Armed with roasted samples of the Guatemala in both High and Low aW samples in all three roasting styles, I collected data from the Roasters Guild Retreat seminar attendees, who tasted blind and submitted data voluntarily. Sixty three respondents used a 0-3 scale to rate sweetness, acidity, and viscosity in the six samples, served via batch brewed Bunn coffeemakers.
The data only somewhat corroborated my working theory of Maillard rate and its affect on sweetness, acidity, and flavor. However, it showed that the High aW batch was notable for its higher perceived acidity, which persisted regardless of roast style. The Low aW sample responded very unfavorably to an extended Maillard time, and its acidity tracked predictably down and up during corresponding slow and fast Maillard reaction times. The High aW sample’s sensory attributes remained proportionally less affected by Maillard reaction rate, by comparison.
Ultimately, the High aW sample was less affected by stylistic changes in roasting during the Maillard reaction prior to first crack, particularly in regards to its perceived level of acidity. The Low aW sample was more sensitive to heat changes during the Maillard reaction, perhaps because it was predisposed to a slower reaction rate. More profound sensory changes were observed from roast to roast in the Low aW sample.
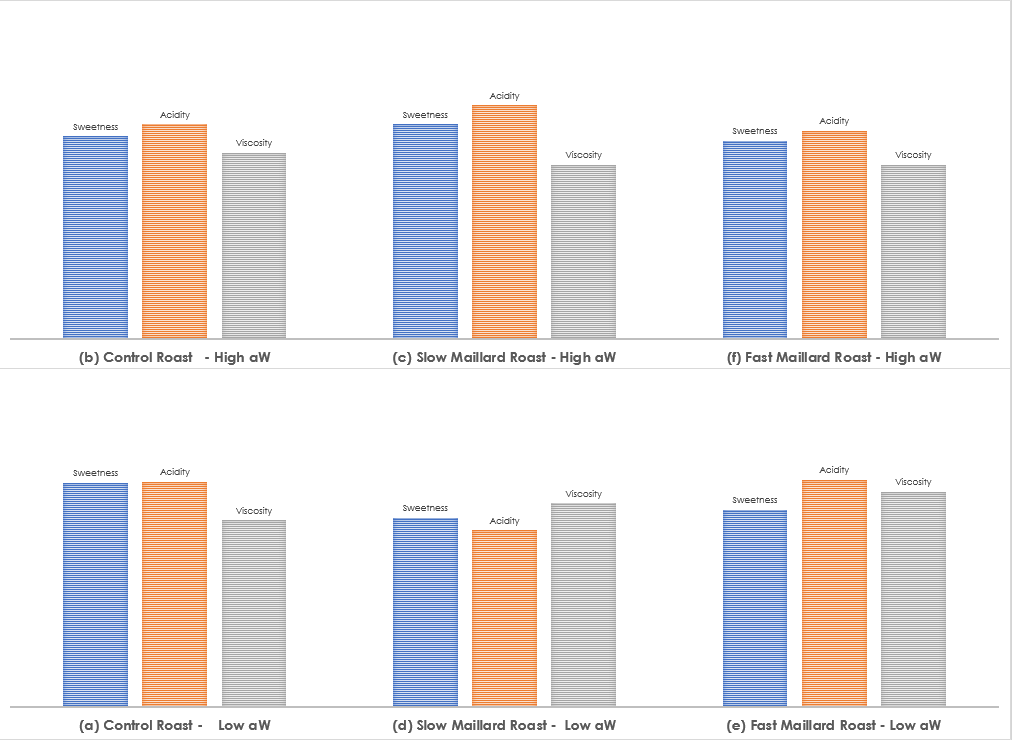
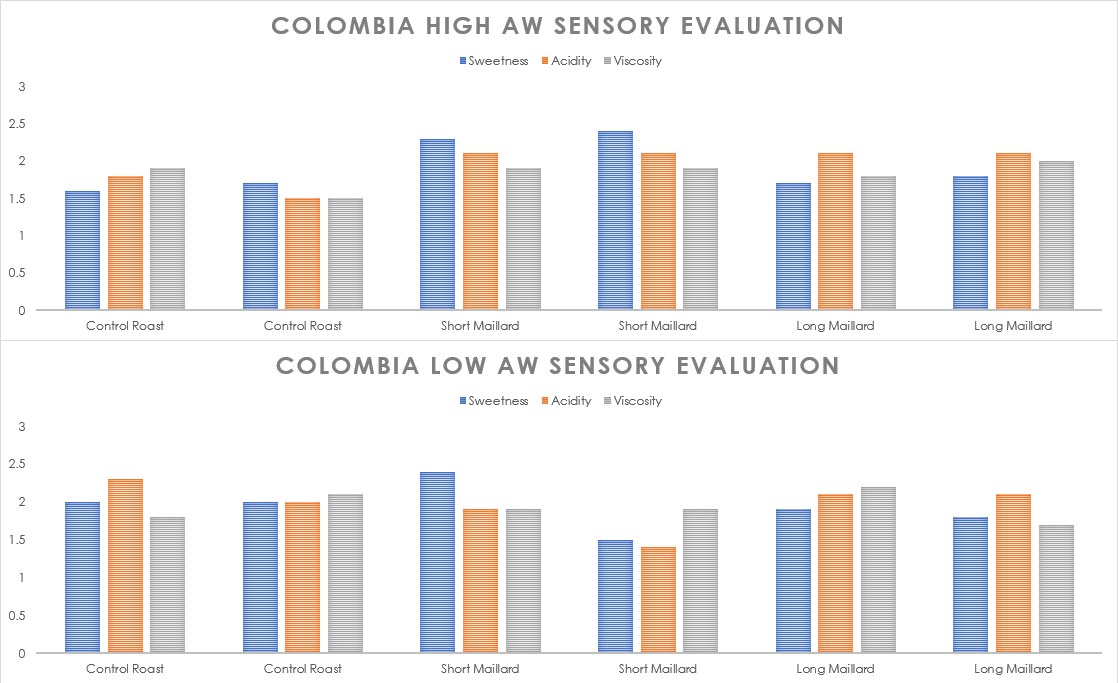
Colombia / Ikawa Sensory Evaluation
The five members of The Crown tasted 12 randomized roasts of the Colombia completely blind, and used the same 3-point rating system to indicate intensity of sweetness, acidity, and viscosity. I’ve averaged the results together below:
Once again, strong positive reactions to the shorter/fast Maillard rate in the High aW sample. Otherwise, the coffee was generally less predictable than the Guatemala in regard to its sensory response to accelerated or decelerated Maillard reaction rate. However, trends are more predictable in the High aW sample, where shorter Maillard roasts produced sweet, bright coffees while longer Maillard rates reduced the perceived sweetness but not the perceived acidity.
The Takeaway
In general, Jen Apodaca and I have enough observed data and anecdotal evidence to believe that there are at least some indications of overarching principles that can guide our roasting and tasting with regard to the relationship of Water Activity and the Maillard reaction.
- The rate of Maillard reaction can affect a coffees perceived sweetness, acidity, and viscosity. (faster = higher sweetness & acidity / slower = more balanced & more viscosity)
- Water Activity of the green coffee will affect rate and color of Maillard reaction and Sugar Browning.
- Under conditions where, all other things being equal, two coffees of differing water activity are compared, the coffee with higher water activity (approaching 0.70):
- may have an increased rate of Maillard, and may respond more quickly to heat application during this stage of roasting.
- may exhibit acidity that’s (comparatively) resilient to heat/rate of Maillard reaction.
- seems to experience more predictable time/temp of first crack.
- seems to achieve darker colors at similar end time/temp.
What to do as a roaster? Well, the choice is largely yours. Choosing coffees with higher aW may provide you some exciting flavors, but more volatile shelf life. Roasting coffees with higher aW may not need as much heat during the Maillard reaction to achieve balanced flavor profiles. But ultimately, even if we are able to solidify these initial results with further research, there is no substitute for tasting your roasts. Knowledge of water activity and the Maillard reaction can help roasters make informed choices, but will likely never replace their own sensory observations and roasting decisions.
Chris Kornman
Chris Kornman is a coffee romantic and educator, and a quality specialist with a history of indiscreet coffee buying, roaster fires, ill-advised travel, and oversharing. He is the author of Green Coffee: A Guide for Roasters and Buyers and regularly contributes coffee-related disquisitions to publications worldwide.
Comment
4 Comments
Comments are closed.











I’e been aware for some time now that, as a given coffee ages, the water content and water activity both decrease. I’ve also been playing around a fair bit with changes in roast profiles, springing from some of the work of both Jen Apodaca and Roob Hoos, in some experiments to learn more about roasting of older stocks, some even far past their prime. All the recent writings on tis topic, along with my own curiosity, have me wondering if there mightn’t be some protocol for restoring at least part of the water content and activity to bring a tired old dog of a coffee to heel and salvage some of the former goodness lost. Any ideas, thoughts, etc, on how one might go about this?
You could rinse your coffee in water a day or two prior to roasting. It might help a little, but you’re not really restoring the lost organic compounds in the aged coffee… like how you could re-hydrate dried fruit but it’ll never taste the same as if it were fresh. Preventative measures (hermetic bag liners, climate control, roasting soon after harvest, etc.) are far more effective.
Your points are very valid, Chris. I’ve played a bit with a quick rinse or short soak for maybe a quarter of the weighted batch for roasting, but had issues with the wet beans sticking in the chute on the way to the drum. I’ve thought about a longer soak then removing a day or so before roast to at least let the standing water on the surface dry up somewhat.
Of course, the preferred protocol would be to maximise the best flavours in the coffee by proper storage and using it much sooner after harvest. But sometimes there are a couple of good coffees that did not find their way into someone’s cup within optimal time frame, and I’m playing with ways to reclaim as much as might be left in them. I know they will never reclaim all that was in them when first scooped off the warehouse floor in jute. I am going on the premise that there is enough remaining in some of those coffees to warrant a higher use than seasoning or learning the response times of a new roaster. Or as firestarter in the woodstove at home. The little playing I’ve done so far has made some nasty coffees into some acceptable ones. Enough promise to warrant further playing when time permits
wáter activituy wonderfull, i´m a chemical engeneering….plesa send info