In the first half of this series, we took a shallow dive into the deep pool of information on the chemistry and composition of water, while exploring what kinds of options coffee professionals have at their disposal for conditioning that water.
In Part II, I’d like to focus on the sensory data collected specifically at the Crown, and what kinds of conclusions we can make about water formulas and coffee flavor.
Sensory Impact & Safe Range
The importance of safety shouldn’t be undervalued, but ultimately most roasters and baristas are interested in making their coffees taste the best they possibly can. Fortunately, there is substantial overlap between making your coffee taste good and keeping your machines alive.
Alkalinity
The space between corrosion and scale formation allows for a fairly wide range of mineral concentration but a narrow range of alkalinity. In terms of bicarbonate levels, the SCA Water Quality Handbook suggests that levels below 40 ppm (as CaCO3) can result in too little buffer to prevent corrosion at boiling temperatures. However, high levels of bicarbonate are not generally very tasty. SCA posits an upper range of 75 ppm (alkalinity as CaCO3) when using harder water levels, while Water for Coffee suggests that around 80 ppm bicarbonate HCO3– (about 130 ppm alkalinity as CaCO3) could be appropriate with harder waters.
In either case, high amounts of bicarbonate can result in lower perceived acidity in coffee and in extra carbon dioxide — impeding extraction and making the bloom or crema bigger.
Frighteningly, it doesn’t take much to get out of range. If, for example, you’re using off-the-shelf baking soda, just 0.1g in 1 liter of distilled water will get you about 50 ppm (alkalinity as CaCO3) — as Goldilocks would say, “just right.” But if you edge up to 0.2g in a liter, you’ve jumped to 100 ppm (alkalinity as CaCO3), which is probably more than you need in all but the very hardest of waters.
I spoke recently with Taylor Minor, CEO of Third Wave Water, because I noticed that the “Classic” water profile contains no bicarbonate alkalinity at all. He said this was determined primarily at the cupping table. The recommended alkalinity spec, in his opinion, was “indicative of most average water profiles across the US, and one that won’t destroy your espresso machine.” But because Third Wave Water exists as a niche customization option, their one-gallon-at-a-time mineralization model needn’t be held hostage by alkalinity.
In our testing, high alkalinity water resulted in very flat, chalky, coffee with muted acidity and muddled flavors. A lower ratio, about 1 part alkalinity for every 2 to 3 parts hard minerals (as CaCO3), provided better results. Waters with only alkalinity, even in the “ideal” range, lacked the right minerals to extract coffee’s sweetness in our cuppings, though they performed at their best when applied to darker roast styles.
Calcium
The most abundant naturally occurring hard mineral in most waters is also pretty great for coffee. What luck!
Calcium is a good extractor, bonding readily with coffee acids. However, there is a risk of limescale formation. Scale forms more easily at higher temperatures, so a pressurized steam boiler in an espresso machine is at greater risk than a hot water tower, for example. This also depends greatly on how much new, unheated water flows into the system.
Regardless, given that calcium is typically the common hard mineral naturally found in our water sources, it makes some sense to examine its qualitative effect on coffee brewing. In our trials, calcium-formulated water has been consistently favored by the majority of our panel. It accents coffee’s acidity in enjoyable ways regardless of processing method or roast level. In dark roasts, the calcium-rich water maintained a presence of acidity best when compared to other mineralized options — a characteristic rewarded by our panel, but perhaps not always desirable to dark-roast drinkers.
This feels like a good time to remind ourselves that sometimes preference can be correlated with acclimation. In this case, it’s possible that because the calcium-formulated water most closely resembled what we normally drink, it also produced the favorite profile — not because it was objectively better, but because its was what we were accustomed to drinking.
Magnesium
Christopher Hendon and Maxwell Colonna-Dashwood tout magnesium as a miracle mineral. They were able to demonstrate that it was more efficient at extraction, without the risk of limescale. They also liked its flavor profile.
Naturally occurring magnesium-rich water is rare, so to achieve it often requires customization. However, because of magnesium’s low molar mass and low concentration in available chlorides and sulfates, adding it to water can raise the risk of corrosion to equipment.
Our taste panelists found plenty of positive characteristics, including lots of sweet and floral attributes, particularly in lighter roasts. However, especially as roasts darkened, many tasters found the mouthfeel overwhelming, and the sweetness cloying.
For us, magnesium seems like a good “accent” mineral in terms of flavor. A pinch can bring out some nice sweetness, and when paired with calcium it can help to balance a coffee’s profile without being invasively sweet or sticky.
Quality Results
We engaged in two well-documented rounds of taste-testing, using different mechanisms for recording our data. My tasting panel included the whole Crown team: Sandra Loofbourow, Jen Apodaca, Evan Gilman (all Q graders), Richard Sandlin, and myself.
Round 1
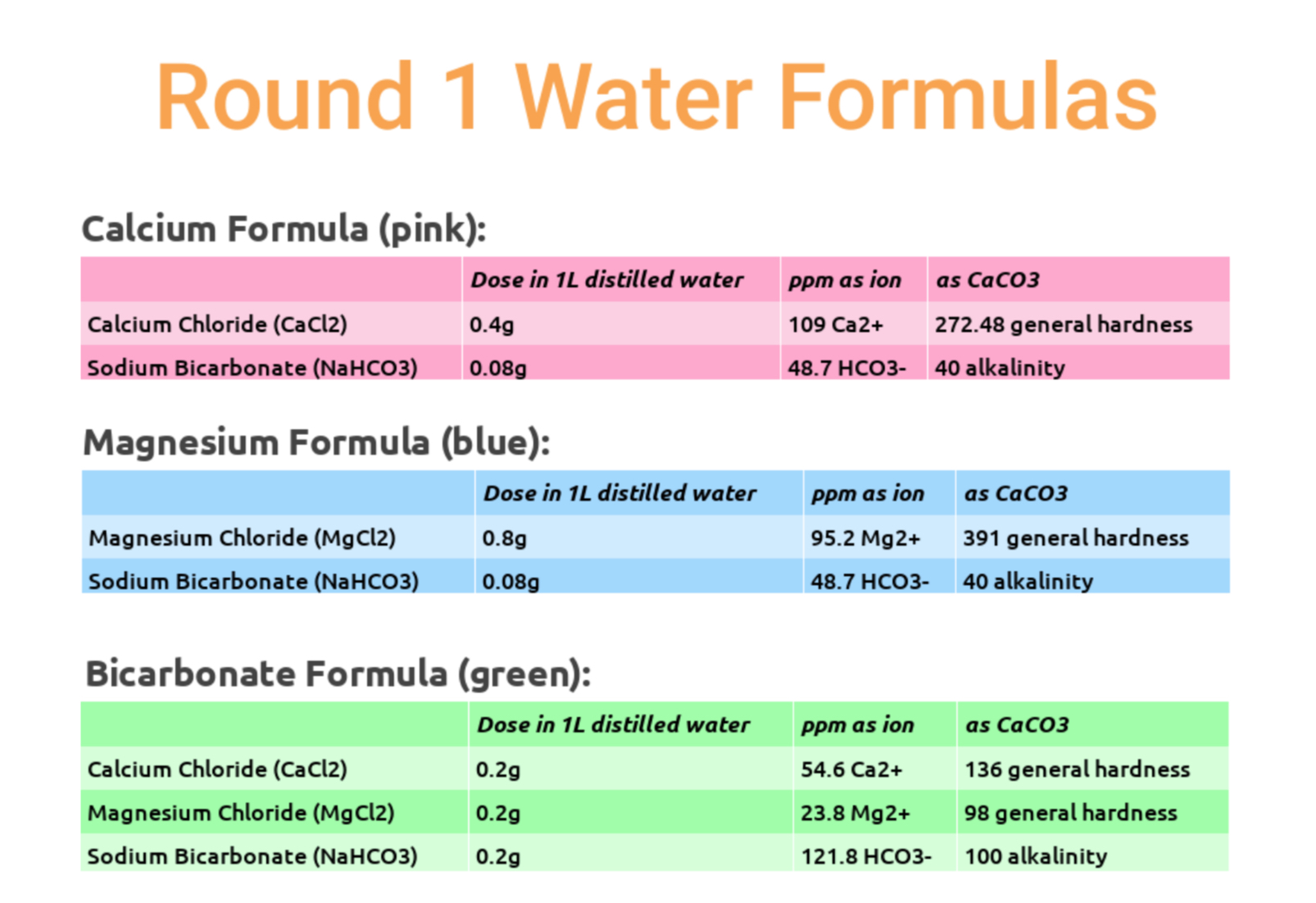
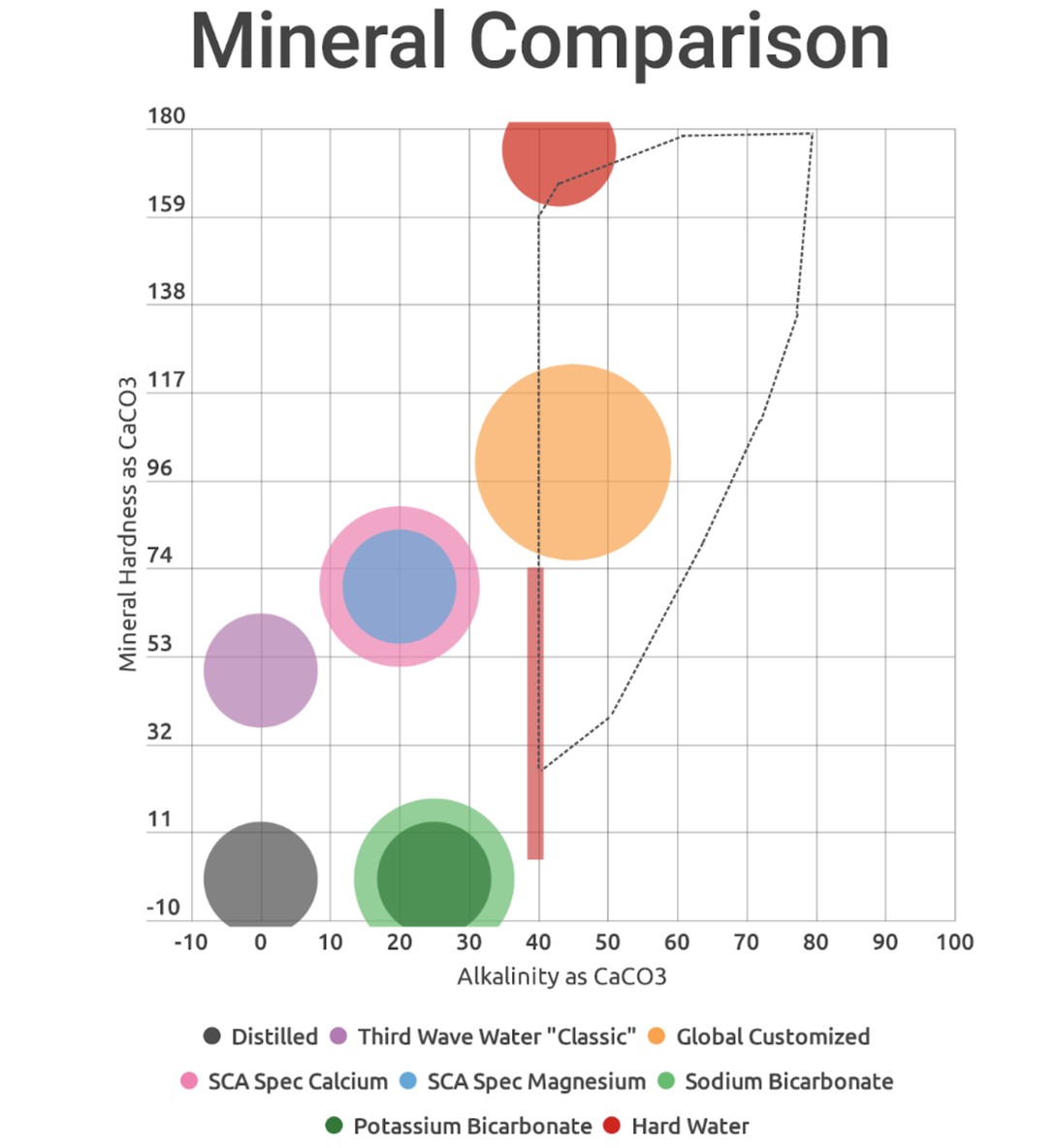
Our first round of testing involved three different roast levels (~55 Colortrack Light, ~65 Colortrack Medium, ~70 Colortrack Dark) of two different coffees: a natural and washed Ethiopian selection. Three water formulas were in play, all very high in mineral content in an attempt to accent the extreme top end of what each mineral might contribute to flavor, using a calcium formula (pink), a magnesium formula (blue) and a high bicarbonate formula (green). You can see the exact recipes below, but note that these are not recommended, for reasons related to corrosion and limescale formation.
The cupping was not completely blind; color codes were revealed, as were roast levels, so the only mystery to cuppers was what the colors represented. We took notes, and ranked preference. No sensory scores were taken.
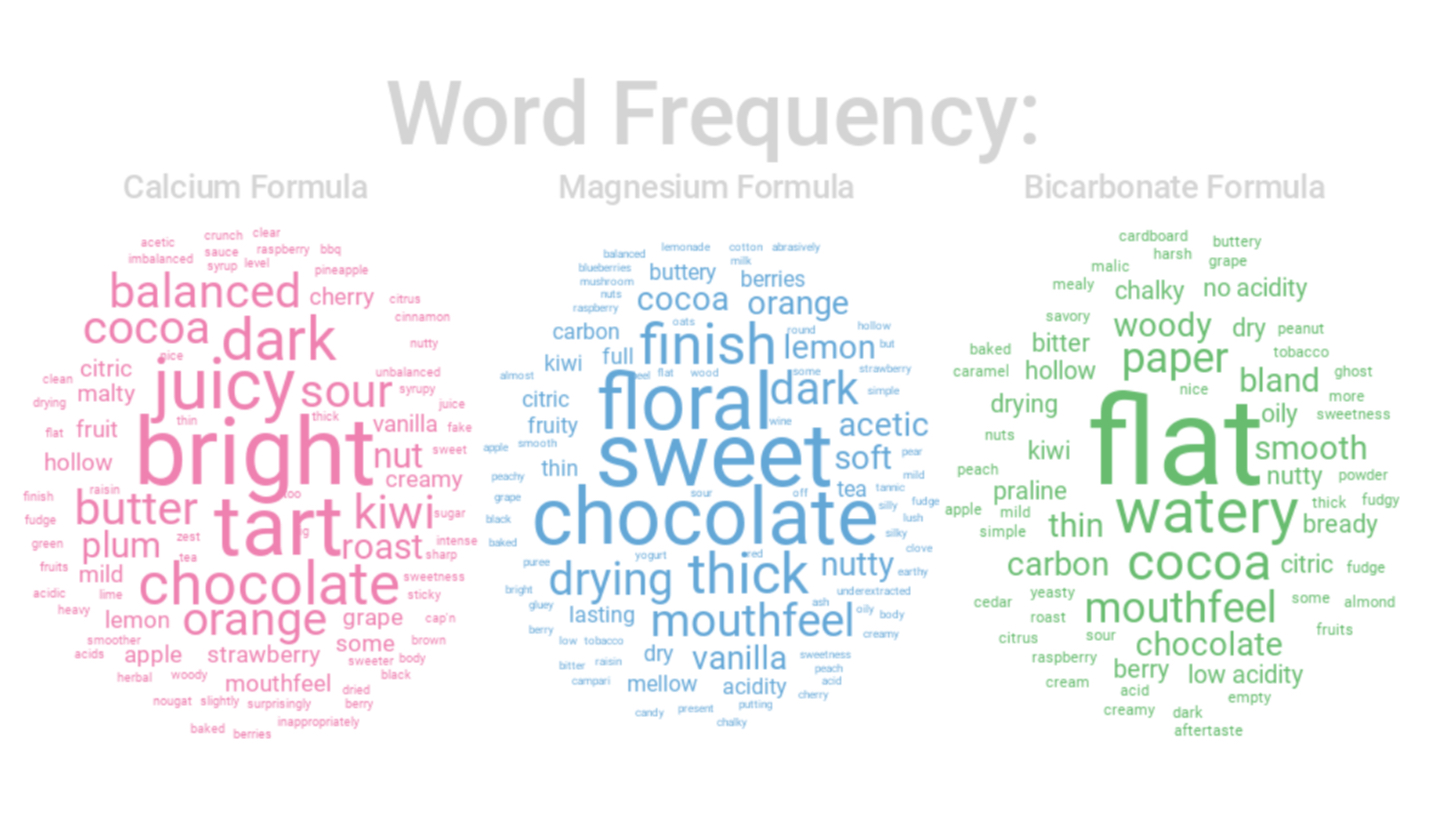
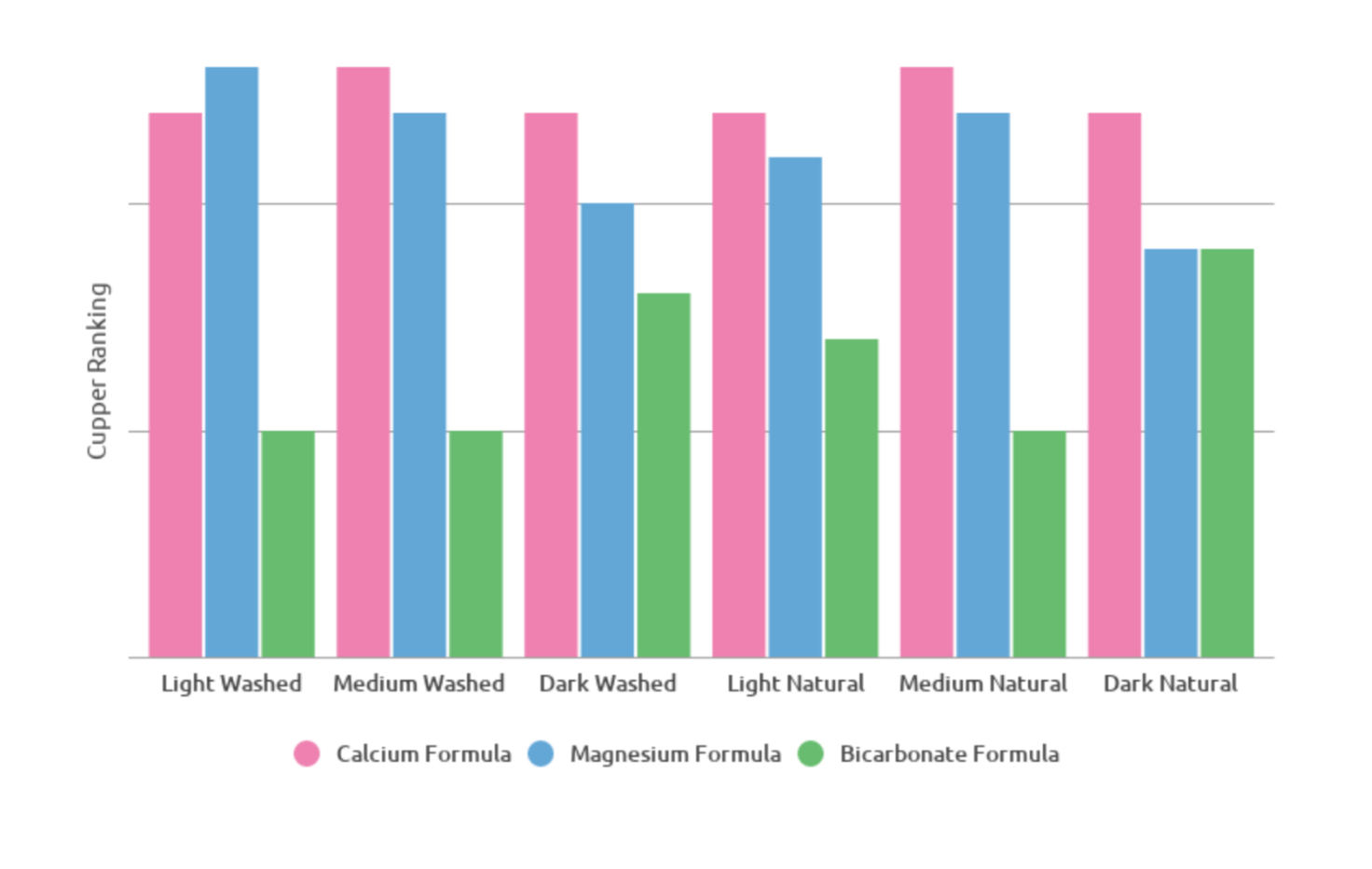
In all but the light roasted washed coffee, the calcium formula edged the others in overall cupper preference, though magnesium kept a close pace, mostly at lighter roast levels. You can see in the word clouds that there were significant qualitative differences produced by different formulas produced; the calcium tended to focus on acidity, while magnesium was sweeter, more floral and thicker.
The bicarbonate formula, by contrast, restricted acidity, muted flavors, and left a chalky mouthfeel. It did, however, seem to perform a little better when applied to darker roasting styles — a curiosity that might be worth noting for those who enjoy coffees taken to second crack.
Round 2
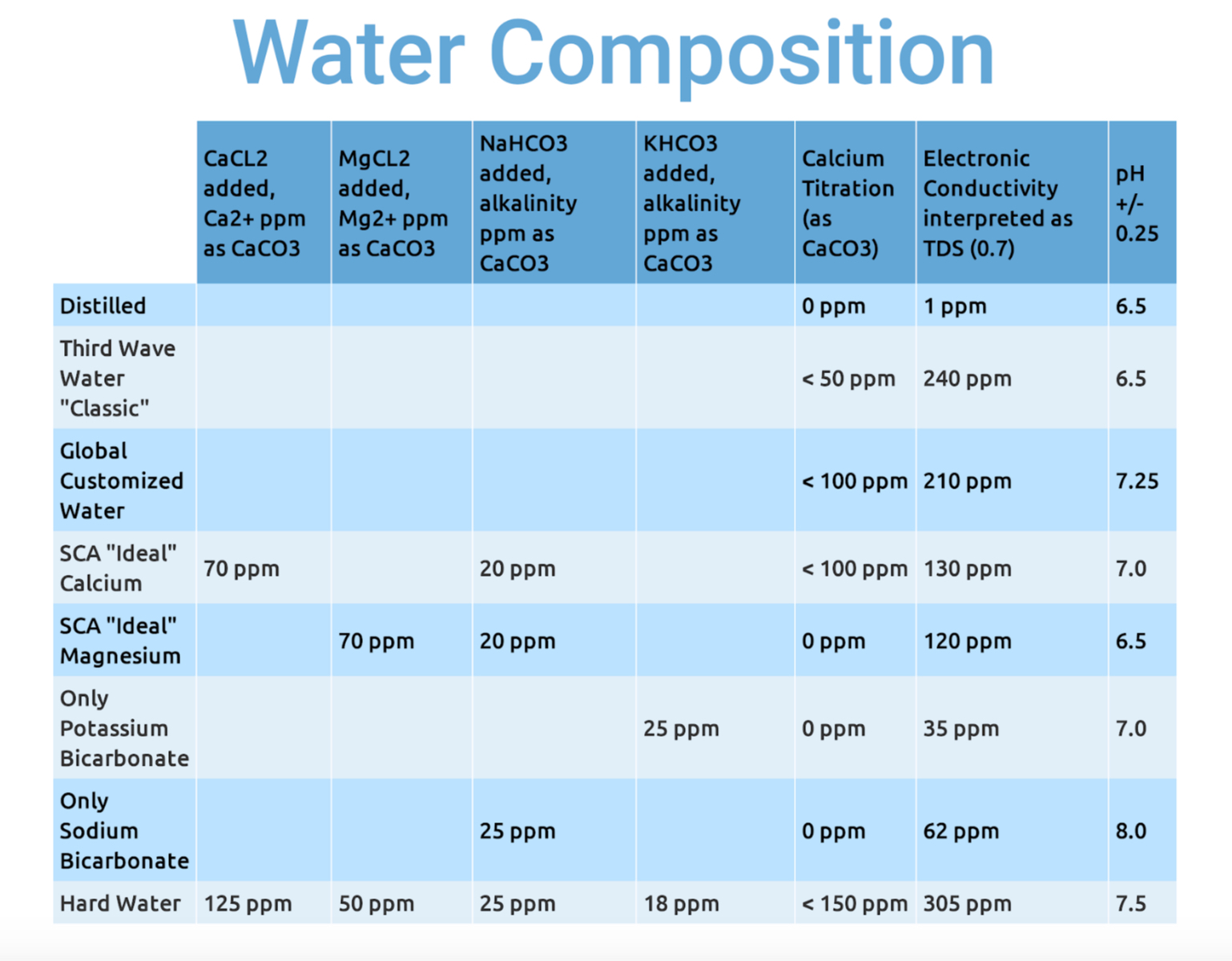
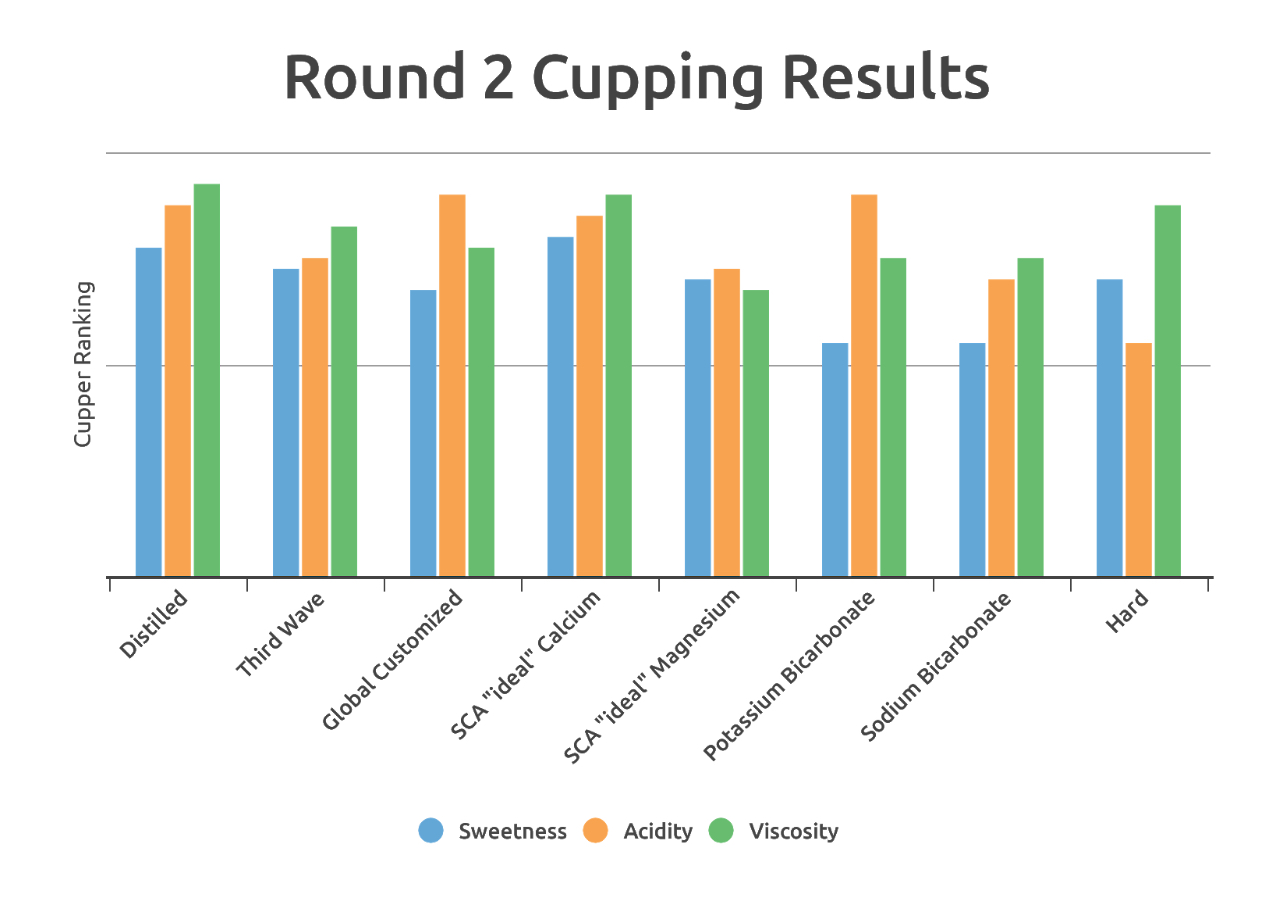
The second round of testing narrowed the coffee variable to a single roast of a high scoring Mexican coffee, and explored a range of water formulas and their effect on acidity, sweetness, and viscosity. Here are the water compositions:
You’ll no doubt observe some oddities. The conductivity meter, interpreting as TDS, seems only vaguely reliable. My titration kit, designed for aquariums at Ca2+ concentrations around 400 ppm (about 1000 ppm as CaCO3), was only accurate within a relatively wide margin of error. And there’s always the possibility that when adding minerals in 100th of a gram increments, some small error in mass calculation could result in a large swing in TDS.
Note the pH readings. Baking soda did indeed result in the most alkaline solution, while Magnesium even with a buffer and Third Wave Water with zero alkalinity, had no measurable effect on acidity.
Here’s the visualization, with SCA heritage water spec (red dash) and Colonna-Dashwood/Hendon (dotted lines) overlaid. You’ll note that my customized formulas fall slightly lower than intended on alkalinity, due to an error in calculations.
Below, qualitative results can help to clarify a picture of what effect each mineral, and its relative concentration level, contributed to overall flavor. The ratings are grouped by sample, averaged by attribute, rated on a scale of 0-5. The cupping was double-blind to help prevent bias.
A number of interesting observations here are worth discussing. Waters with only bicarbonate and no hard minerals had the lowest sweetness, by a wide margin. The sodium bicarbonate also resulted in a significantly thicker and more bubbly crust.
Surprisingly, distilled water performed very well, earning the highest tallied point score as well as and top ratings from both Richard and Jen. It presented a balanced acidity, decent sweetness, and a silky, smooth mouthfeel.
Jen wondered aloud if perhaps less is more; maybe too many compounds in water just get in the way of flavor. Further evidence to support this came in the form of hard water, which rated lowest of the table in acidity but near the top in viscosity — thick and muddy, a good cup for a truck stop, perhaps, but not ideal for a specialty roaster.
Formulated waters by Global Customized Water and Third Wave Water struck a nice balance. Jen and Sandra rated the Third Wave “Classic” profile quite highly, while Evan and I were impressed by the Global Customized brew. Global Customized Water seemed to produce a more acidic coffee, likely due to its calcium content, while the Third Wave extraction seemed a little more focused on viscosity, probably a result of the inclusion of magnesium in the mineral blend.
Lastly, custom calcium and magnesium “ideal” spec coffees matched flavor expectations from the previous round of testing. Calcium water produced more acidic and across the panel high ratings, second by just a hair to the distilled water in total point score. Magnesium rated much lower — except for Sandra, who magnanimously gave elevated scores accompanied by a definitive “but I don’t like it” in her notes — with three of the five panelists using the term “flat.”
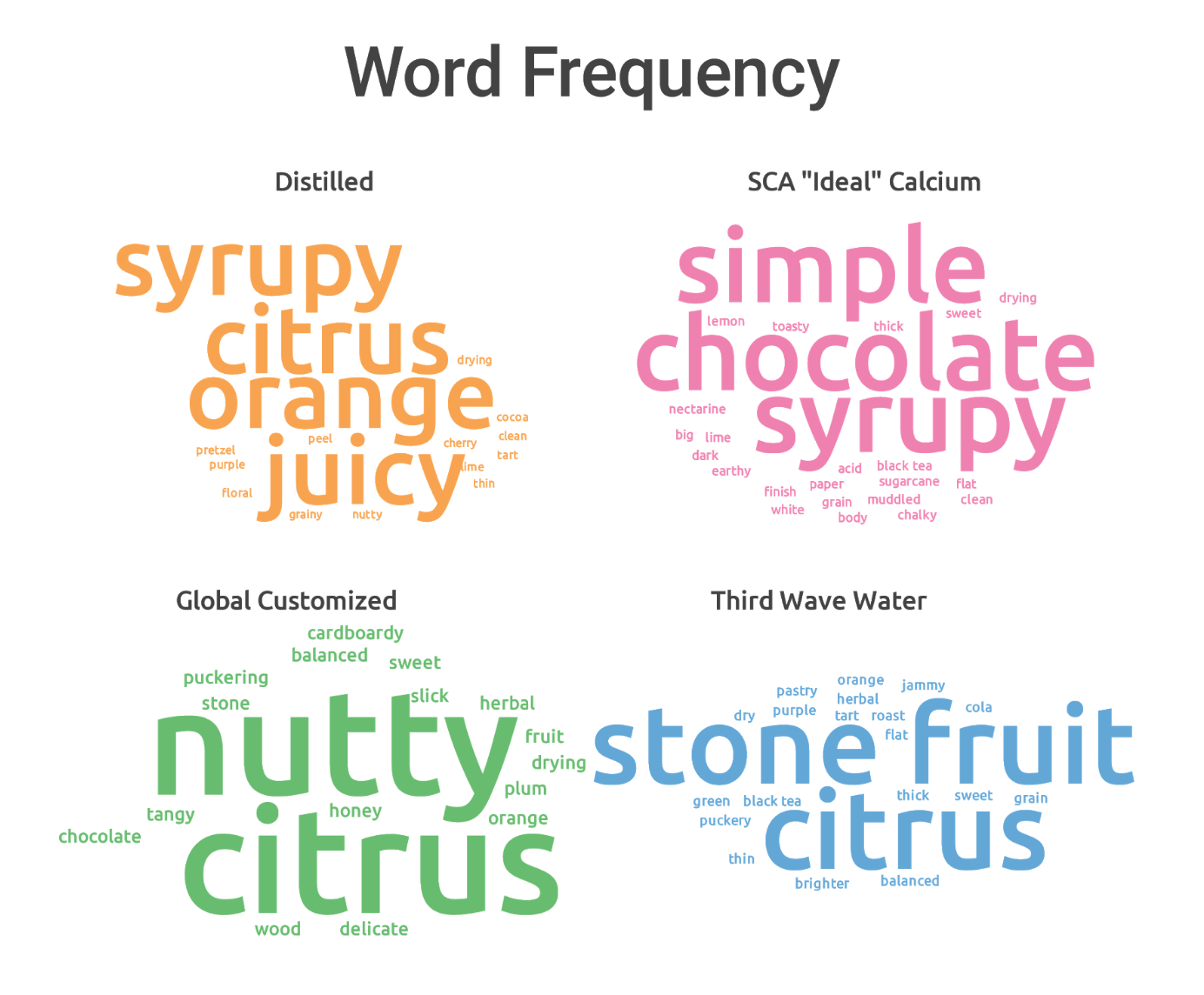
Of course, points aren’t everything. A comment Jen made about the SCA “ideal” calcium water resonated with me: While that water looks great by the cupper ratings, it felt a little bland in terms of flavor expression. It “checked all the boxes” in terms of acidity, sweetness, and viscosity, but in terms of pleasurability of taste, it didn’t hold up as well. Overall, it was the distilled, Third Wave, and Global Customized waters that seemed to have a nicer looking overall set of descriptors. Here’s the word cloud for the top four:
Conclusions
Ultimately, we found that there are many good solutions available, but no perfect answer to making great coffee. Whether working with what’s streaming through the taps, or customizing water systematically or in small batches, the choices will largely depend on roasting style and the people who are making the quality-control decisions, who must continually balance taste and safety.
Hopefully all this chatter about bicarbonates, alkalinity, calcium, and magnesium hasn’t muddied the waters, but instead provided a (relatively) concise and approachable guide for people interested in making great coffee with the resources at their disposal. I’d encourage you to craft some trials on your own, and see what kinds of flavor possibilities you can bring to the surface.
Chris Kornman
Chris Kornman is a seasoned coffee quality specialist, writer and researcher, and the Lab & Education Manager at The Crown: Royal Coffee Lab & Tasting Room.
Comment
2 Comments
Comments are closed.







I’m shocked by the high scores given to distilled water!
With a Phd in Biochemistry, I understand all the implications of TDS and the usable anions of Ca. I have also messed around with adding Mg in the form of Epsom salts, etc. My tap water comes from a rural approved source that has a TDS around 680 ppm. It is horrible for any use, therefore we run it through a softener (Culligan) . That water has a TDS of 770 ppm, not surprising. For drinking, the ion exchange water is purified by RO filtration to yield a water of TDS 28 ppm. I make coffee from this source. It is wonderful! If I adjust this water to 50 ppm Mg it is good but tastes a little brackish. The TDS is about 80 ppm the sum of the RO TDS and what is added with Epsom salts. After all my piddling around with Mg, I have concluded that TDS is as important as cation identity. When TDS gets above 50 ppm taste change occurs. Has any one commented on the effect anion identity?. After all, CaC03 and CaCl2 are two different molecules. Anyway, my “taster” isn’t sensitive enough to comment. In the few tasting test in which I have participated, my comment usually is “it taste like real good coffee”! Culligan tells me that RO only removes 98 to 99 % of the TDS of any tap water. I think maybe the addition of Mg as MgSO4 serves to titrate the water to optimal TDS. I have decided to stick with RO water and forget the salts.